Practical: GROMACS + CP2K Part III
Overview
Teaching: 15 min
Exercises: 60 minQuestions
How to build up QMMM system for protein from the PDB file?
How non-standard CP2K input parameters could be specified?
Objectives
Make protein QMMM system starting from the PDB structure
Usage of non-standard CP2K input parameters
Cacluation of the absorption spectra for your system
Preparing for the tutorial
Everything, which is written inside the gray box are a commands, that should be executed in the terminal window, string-by-string, each following with the ENTER button.
Please note that <...> in the commands means, that everything, including <> symbols, must be replaced with your own specific information. Be careful!
Helpful utilities and commands
Some exercises will require usage of
lessLinux tool for looking up into the content of files. In case you are not familiar with it, here is a short list of hotkeys, which could be used inside LESS editor:
- q – exit
- / – search for a pattern which will take you to the next occurrence
- n – for next match in forward
- N (SHIFT+n) – for previous match in backward
- g – go to the start of file
- G (SHIFT+g) – go to the end of file
All exercises will require you to submit job for computing using
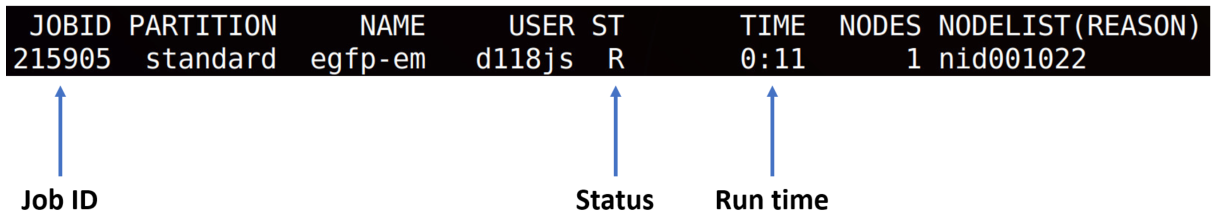
sbatch run.shcommand. To check status of your job following commands would be useful.
squeue -u <your login name>- checks status of all your jobs. Output will look like that:scancel <JOBID>will remove the job, if you occasionally submitted it.
Setting up tutorial environment
Let’s start the tutorial with the following steps
- Execute commands in the terminal:
module load gromacs-cp2k
cd /work/ta025/ta025/<your login name> - And go to the tutorial directory
cd tutorial
Exercise 5: Setting up simple protein system starting from the PDB file
1) Go to egfp directory:
cd egfp
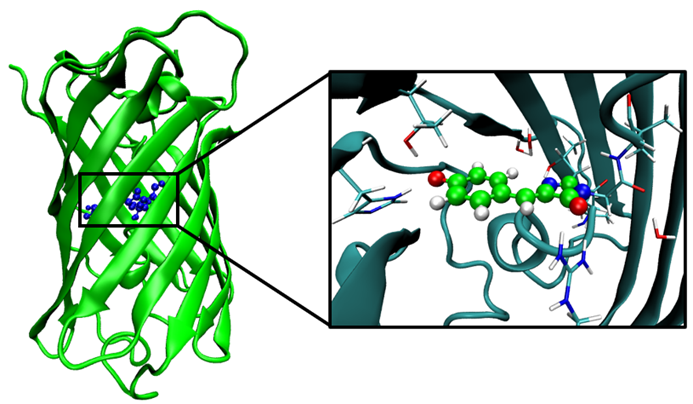
In the directory located forcefield and 4eul.pdb file with a strucutre of EGFP protein dowloaded from PDB databank.
Note that file was modified, missing atoms have been added, first and last residues has been marked as N- and C- terminus, periodic box has been added 10 x 10 x 10 nm.
In addition forcefield for non-standard Chromophore residue CRO66 has been generated with Antechamber.
You can download it and inspect structure with VMD or PyMOL.
2) Make topology for the system using the following command:
gmx_cp2k pdb2gmx -f 4eul.pdb
choose the following forcefield and water model:
Select the Force Field:
From current directory:
1: AMBER03 : Neutral GFP
....
....
Select the Water Model:
1: TIP3P TIP 3-point, recommended
Files topol.top, conf.gro and posre.itp should appear in the directory
3) Solvate te system in the conf.gro
gmx_cp2k solvate -cp conf.gro -o conf.gro -p topol.top -shell 10
4) Now we need to make our system neutral by adding 6 Na+ ions
To do that first generate tpr file:
gmx_cp2k grompp -f em.mdp -p topol.top -c conf -o egfp-genions.tpr -maxwarn 10
then use the following command to replace 6 random water molecules with Na+ ions
gmx_cp2k genion -s egfp-genions.tpr -p topol.top -o conf.gro -neutral
select group 13 of SOL molecules Select a group: 13
after that manipulations your conf.gro and topol.top files will contain solvated and neutralized protein system.
5) The next step would be minimization and short classical equilibration NVT trajectory.
First generate and run energy minimization:
gmx_cp2k grompp -f em.mdp -p topol.top -c conf -o egfp-em.tpr
sbatch run-em.sh
Wait until simulation will be completed.
Then perform 100 ps NVT simulation starting from the optimized structure to equilibrate our system:
gmx_cp2k grompp -f md-mm-nvt.mdp -p topol.top -c conf.gro -t egfp-em.trr -o egfp-mm-nvt.tpr
sbatch run-mm-nvt.sh
while simulation is running you could check em.mdp and mm-nvt.mdp files for the details of classical MD simulations
6) Next step would be changing simulation from classical forcefiled to QMMM.
First generate index.ndx file that would contain QMatoms group, marking QM atoms in our protein:
gmx_cp2k make_ndx -f conf.gro
and do the following input
> a 938-956
> name 18 QMatoms
> q
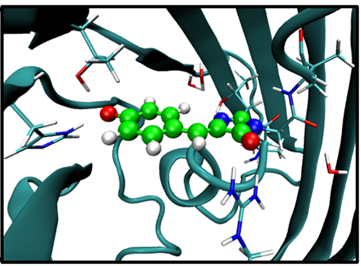
Look into the conf.gro with VMD or PyMOL and make sure that atoms from 938 to 956 are the same as shown in spheres on the following figure:
7) Now we are ready to generate QMMM simulation file:
gmx_cp2k grompp -f md-qmmm-nvt.mdp -p topol.top -c conf.gro -t egfp-mm-nvt.trr -n index.ndx -o egfp-qmmm-nvt.tpr
Here we are using classically equilibrated trajectory egfp-mm-nvt.trr as a starting point for QMMM simulation.
8) Run QMMM simulation:
sbatch run-qmmm-nvt.sh
While simulation is running you could inspect md-qmmm-nvt.mdp and check that QM part in that case has charge -1.
; CP2K QMMM parameters
qmmm-active = true ; Activate QMMM MdModule
qmmm-qmgroup = QMatoms; Index group of QM atoms
qmmm-qmmethod = PBE ; Method to use
qmmm-qmcharge = -1 ; Charge of QM system
qmmm-qmmultiplicity = 1 ; Multiplicity of QM system
9) At the end of the simulation you can download trajectory file egfp-qmmm-nvt.trr and render it using your favorite software (e.g. VMD, PyMOL).
Also you could check temperature as a function of time with the following command:
gmx_cp2k energy -f egfp-qmmm-nvt.edr
and choose 16 Temperature
File energy.xvg will contain data about Temperature (K) against simulation time (ps).
Exercise 6: Using non-standard parameters in CP2K input
1) Stay in the same egfp directory
2) Copy egfp-qmmm-nvt.inp and egfp-qmmm-nvt.pdb files:
cp egfp-qmmm-nvt.inp egfp-qmmm-spec.inp
cp egfp-qmmm-nvt.pdb egfp-qmmm-spec.pdb
3) Modify egfp-qmmm-spec.inp file you have just copied with vim egfp-qmmm-spec.inp or any other editor.
Insert between &END DFT and &QMMM lines an additional &PROPERTIES section.
Final result should look like that:
&END DFT
&PROPERTIES
&TDDFPT
NSTATES 5
MAX_ITER 10
CONVERGENCE [eV] 1.0e-3
&END TDDFPT
&END PROPERTIES
&QMMM
This will order CP2K to also calculate 5 excited states at each MD step with TDDFT.
4) Generate Gromacs-CP2K simulation file:
gmx_cp2k grompp -f md-qmmm-spec.mdp -p topol.top -c conf.gro -t egfp-qmmm-nvt.trr -n index.ndx -o egfp-qmmm-spec.tpr
5) Run simulation:
sbatch run-qmmm-spec.sh
6)While it is running inspect content of md-qmmm-spec.mdp file, the following lines will order GROMACS to use external CP2K input file:
; CP2K QMMM parameters
qmmm-active = true ; Activate QMMM MdModule
qmmm-qmgroup = QMatoms; Index group of QM atoms
qmmm-qmmethod = INPUT ; Method to use
qmmm-qminputfile = egfp-qmmm-spec.inp ; external input file
7) After job is finished, we need to gather information about excitation energies over the calculated trajectory:
grep " TDDFPT|" egfp-qmmm-spec.out | awk '{ print $3 " " $7 }' > excitations
The excitations file should appear in the directory, it will consist out of two columns.
First column is an excitation energy (in eV) and second is an oscillator strength (in a.u.) for each excitation computed by CP2K.
Final absorption spectra could be convolved by representing each excitation with gaussian function and sum up over all of them.
8) Convolve the spectra using provided Python script:
module load cray-python
./conv.py excitations 0.1 2 5
File spec.xvg should appear in the directory. You can open it in Grace or copy data into any other software (i.e. Excel).
As an example, convolved spectra with 0.1 eV half-width gaussians over 100fs (100 steps) trajectory:
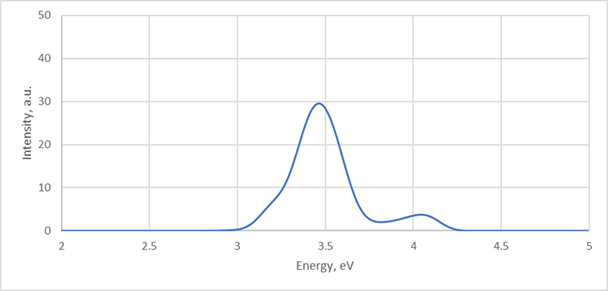
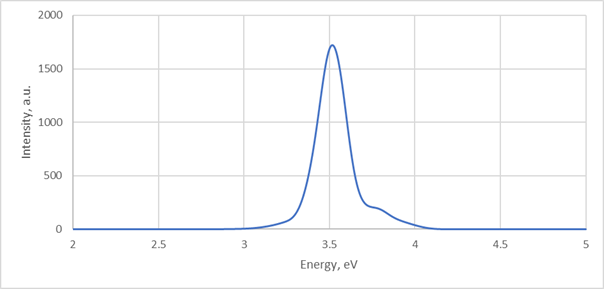
9) Spectra collected over 3 ps (3000 MD steps) will look like that:
Key Points
QMMM system the protein could be built from the classical MD system directly
Non-standard CP2K parameters could be specified directly in the generated CP2K input file
qmmm-qmmethod = INPUT should be used for providing your own CP2K input file
Advanced properties, like absorption spectra could be calculated using external input files